Total hardness
Total water hardness is a measure of the capacity of water to precipitate soap. Soap is precipitated primarily by the calcium and magnesium ions present. Other polyvalent cations may also precipitate soap, but they are often in complex forms, often with organic compounds, and their contribution to water hardness may be minimal and difficult to determine.
In fact, we express water hardness as the sum of calcium and magnesium concentrations in milligrams per liter of calcium carbonate.
Types of total water hardness
1- Carbonate or temporary hardness: This type of hardness is caused by the presence of calcium bicarbonate and magnesium bicarbonate in water. We can temporarily eliminate this type by heating and boiling the water.
2- Non-carbonate or permanent hardness: This type of hardness is caused by the presence of calcium sulfate, calcium chloride, calcium nitrate, and other calcium and magnesium compounds. We cannot remove this type of hardness by heating.
Consequences of water hardness
1- Reduced heat transfer efficiency: Mineral deposits form on heating surfaces such as boiler tubes, heat exchangers, and steam boilers. These deposits provide thermal insulation and reduce heat transfer, resulting in a decrease in the efficiency of the heating system and an increase in energy consumption.
2- Blockage of pipes and equipment: Calcium and magnesium deposits can block pipes and equipment. This can lead to pressure drops, loss of production, and even equipment failure.
3- Corrosion: In some cases, deposits cause corrosion of metal pipes and equipment. This leads to pressure drop, loss of production, and even equipment failure.
4- Reduced product quality: Excessive water hardness can affect chemical reactions and the quality of the final product, both in terms of appearance and physical and chemical properties.
5- Reduced effectiveness of soap and detergents: Hard water reacts with soap and detergents, reducing their effectiveness. This is due to the formation of insoluble calcium and magnesium salts that are produced as foam.
6- Increased costs: Sedimentation and corrosion require constant and expensive repairs and maintenance.
How to measure
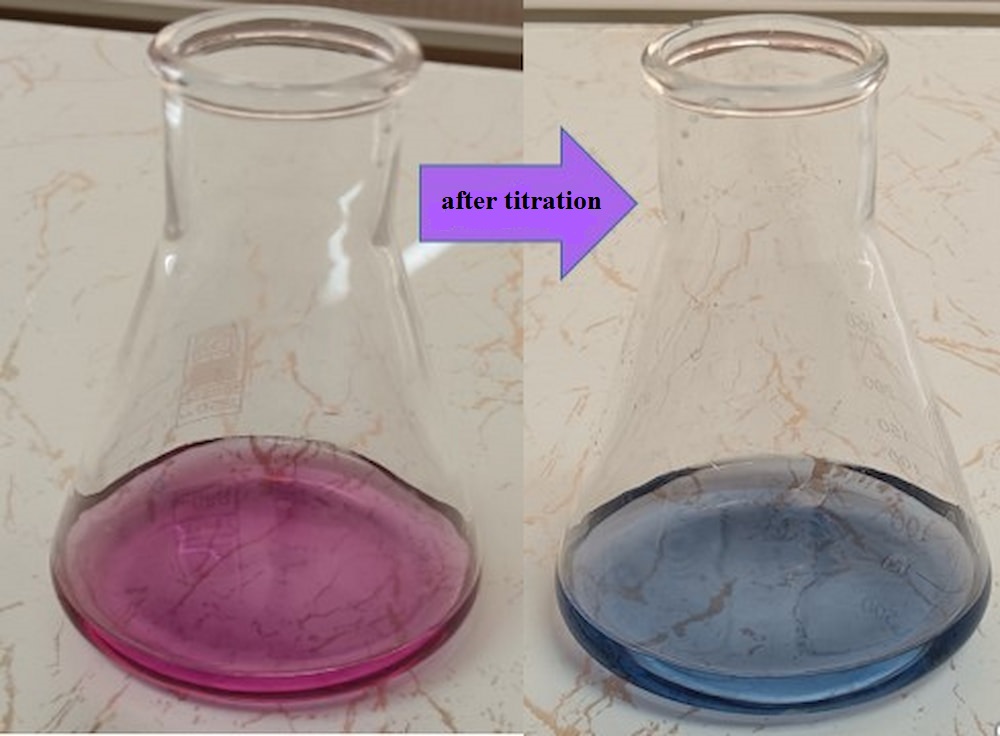
We use the titration method to measure total hardness. Ethylenediaminetetraacetic acid and its sodium salts (EDTA) form a chelated solution complex when added to a solution of certain metal cations. If we add Eriochrome Black T reagent to an aqueous solution containing calcium and magnesium ions at pH=10.0, the solution turns red.
Share: